ANTIMICROBIAL RESISTANCE (AMR) SURVEILLANCE
|
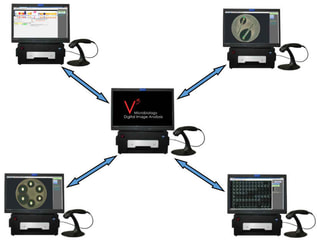
Current AMR surveillance programs sponsored by the US-FDA and CDC use BIOMIC V3 systems to digitally read, interpret, and record standard AMR tests. AMR Testing Modules with BIOMIC V3: Antibiotic Disk Diffusion Zone Reading Broth Microdilution* Plate Reading Etest/MIC Strip Plate Reading Agar Dilution* Plate Reading ID Panel Reading AMR Advantages with BIOMIC V3: - Open system with no proprietary consumables - No routine system maintenance - Portable: Weight: 11kg, Size: 21 x 21 x 14 inches - Operational in extreme environments - Disk ZD & MIC, Strip MIC, Broth Microdilution MIC - Annual CLSI & EUCAST guidelines & expert rule updates - Standardized test procedures and data - Custom epidemiology & QC reports - Verified QC & QA testing - Alerts for unusual, unlikely, or rare test results - Training videos for all procedures - Custom data export formats - Test plate images saved - PC Windows based software - Installation & calibration in less than 1 hour - Software in 13 languages |
AMR Current Programs with BIOMIC V3:
CDC: Carbapenem Resistance (CRE)** Learn More >
CDC: Gonococcal Isolate Surveillance Program Learn More >
US-FDA: Vet-LIRN Veterinary Laboratory Investigation and Response Network Learn More >
US-FDA: NARMS National Antimicrobial Resistance Monitoring System Learn More >
USDA: NAHLN National Animal Health Laboratory Network Learn More >
AMR Previous Program with BIOMIC V3:
From 1997-2010, 151 hospital labs in 40 countries with BIOMIC V3 systems participated in the ARTEMIS drug resistance study sponsored by Pfizer Pharmaceuticals. 50+ journal publications demonstrate BIOMIC V3's testing and AMR surveillance capabilities. View publications >
AMR Multi-Lab Networks with BIOMIC V3:
Multiple BIOMIC V3 systems can be connected on a global network for hospital or private lab groups, government MOH programs, or multiple lab studies. Sites may collect test and QC results to monitor AMR trends from a central location. Study results may be used for regulatory filing, research, or marketing purposes.
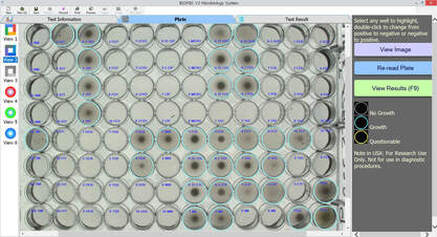
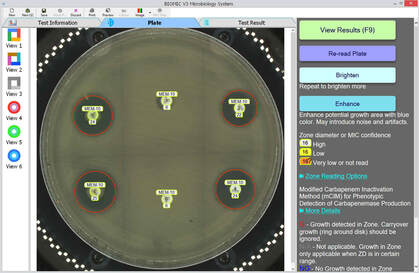
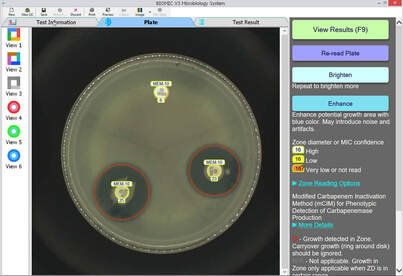
**Samples of BIOMIC V3 Plate Reading for CRE testing:
CDC: Carbapenem Resistance (CRE)** Learn More >
CDC: Gonococcal Isolate Surveillance Program Learn More >
US-FDA: Vet-LIRN Veterinary Laboratory Investigation and Response Network Learn More >
US-FDA: NARMS National Antimicrobial Resistance Monitoring System Learn More >
USDA: NAHLN National Animal Health Laboratory Network Learn More >
AMR Previous Program with BIOMIC V3:
From 1997-2010, 151 hospital labs in 40 countries with BIOMIC V3 systems participated in the ARTEMIS drug resistance study sponsored by Pfizer Pharmaceuticals. 50+ journal publications demonstrate BIOMIC V3's testing and AMR surveillance capabilities. View publications >
AMR Multi-Lab Networks with BIOMIC V3:
Multiple BIOMIC V3 systems can be connected on a global network for hospital or private lab groups, government MOH programs, or multiple lab studies. Sites may collect test and QC results to monitor AMR trends from a central location. Study results may be used for regulatory filing, research, or marketing purposes.
**Samples of BIOMIC V3 Plate Reading for CRE testing: