IQ OQ PQ VALIDATION
|
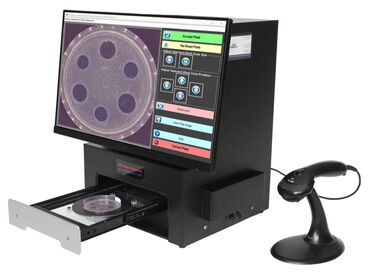
Installation Qualification (IQ), Operation Qualification (OQ) and Performance Qualification (PQ) validation documents are included with each TRINITY V3 purchase. These documents comply with 21 CFR Part 11, Association of Analytical Communities (AOAC), Good Laboratory Practice (GLP), and US-FDA Bacteriological Analytical Manual (BAM). Validation protocols may be customized to meet each laboratory's unique certification requirements.
TRINITY V3 strictly complies with 21 CFR Part 11 electronic signature requirements from reading test plates to reporting test results. Learn more > |
Customer Testimonials - IQ OQ PQ Validation
"Significant labor savings and simple validation with TRINITY V3"
"TRINITY V3 has been an excellent addition to our laboratory. The IQ, OQ, PQ qualification was easily completed within a few weeks. Since implementation, the efficiency in our laboratory has increased significantly in comparison to our old system. We no longer lose a whole day to reading plate assays.
One of the reasons the qualification was smooth was because of the excellent customer service. Any small bump along the way was answered immediately and thoroughly by a team of efficient and dedicated individuals. The team was always available to assist our individual needs."
Laura Olson
QC Manager
Pennfield Animal Health, Omaha, NE, USA
TRINITY V3 customer since 2011
"Fast setup and validation of TRINITY V3 Zone Reader"
"It has been pleasure working with the Giles Scientific sales and service team as we implemented the TRINITY V3 zone reading system for our USP antibiotic potency assays. Their qualification documentation was very simple and straightforward, and the support we received has been great. The system was completely implemented in just a few weeks and we’ve had nothing but praise from the analysts in the lab. The TRINITY V3 system has been very reliable and much easier to use than our old system. We’re looking forward to many years of working with Giles Scientific."
Lab Manager
Michigan-Based Pharmaceutical Manufacturer, USA
TRINITY V3 customer since 2009
"TRINITY V3 has been an excellent addition to our laboratory. The IQ, OQ, PQ qualification was easily completed within a few weeks. Since implementation, the efficiency in our laboratory has increased significantly in comparison to our old system. We no longer lose a whole day to reading plate assays.
One of the reasons the qualification was smooth was because of the excellent customer service. Any small bump along the way was answered immediately and thoroughly by a team of efficient and dedicated individuals. The team was always available to assist our individual needs."
Laura Olson
QC Manager
Pennfield Animal Health, Omaha, NE, USA
TRINITY V3 customer since 2011
"Fast setup and validation of TRINITY V3 Zone Reader"
"It has been pleasure working with the Giles Scientific sales and service team as we implemented the TRINITY V3 zone reading system for our USP antibiotic potency assays. Their qualification documentation was very simple and straightforward, and the support we received has been great. The system was completely implemented in just a few weeks and we’ve had nothing but praise from the analysts in the lab. The TRINITY V3 system has been very reliable and much easier to use than our old system. We’re looking forward to many years of working with Giles Scientific."
Lab Manager
Michigan-Based Pharmaceutical Manufacturer, USA
TRINITY V3 customer since 2009
|
Accessories & Features:
Peni Cylinders Peni Cylinder Dispenser 21 CFR Part 11 IQ OQ PQ Validation Training & Support |