21 CFR PART 11 COMPLIANCE
|
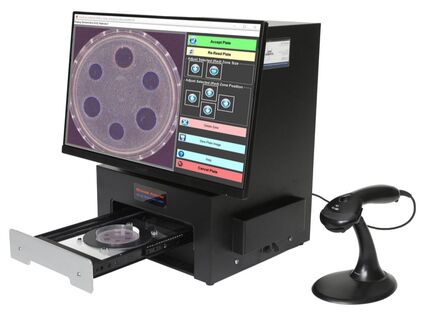
TRINITY V3 strictly complies with 21 CFR Part 11 electronic signature requirements from reading test plates to reporting test results including:
TRINITY V3 settings can be customized to meet your laboratory compliance requirements. |
Customer Testimonial - 21 CFR Part 11 Compliance
"TRINITY V3: A reliable instrument for our USP antibiotic potency assays"
"The TRINITY V3 is flexible and very reliable for our USP antibiotic potency assays. The optics produce high quality images and the software interface is easy to use and navigate. It allows for visual verification of the inhibition zones on screen. TRINITY V3 is also fully compliant with 21 CFR Part 11 requirements."
Tom Dougherty
Fresenius Kabi
Grand Island, NY, USA
TRINITY V3 customer since 2007
Click here to view all Customer Testimonials
|
Accessories & Features:
Peni Cylinders Peni Cylinder Dispenser 21 CFR Part 11 IQ OQ PQ Validation Training & Support |