QUALITY CONTROL & INVENTORY MANAGEMENT
|
BIOMIC V3's automated QC testing and Inventory Management features streamline QC and provide electronic documentation of results. QC and Inventory Management software is included with the following modules: Disk Diffusion, Broth Microdilution, MIC Strip, Agar Dilution, Organism Identification.
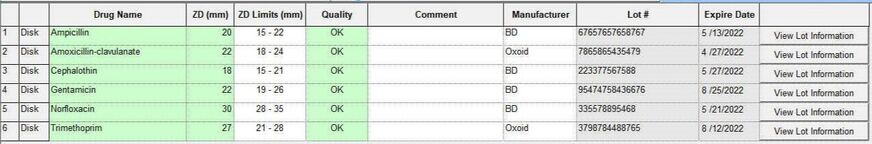
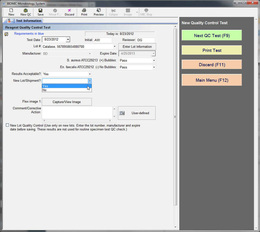
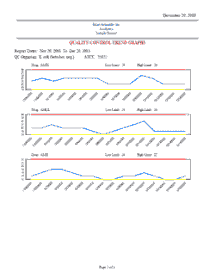
Automated QC Testing Features:
|
Inventory Management
|
BIOMIC V3 provides electronic Inventory Management of:
Record: Entry Date, Material, Quantity, Manufacturer, Lot Number, Expiration Date, In-Use Date, Out-Use Date, Received By, Visual Inspection, Comments, and Custom Defined Fields Sort and print inventory by date received, expiration date, in-use date and out-use date. |
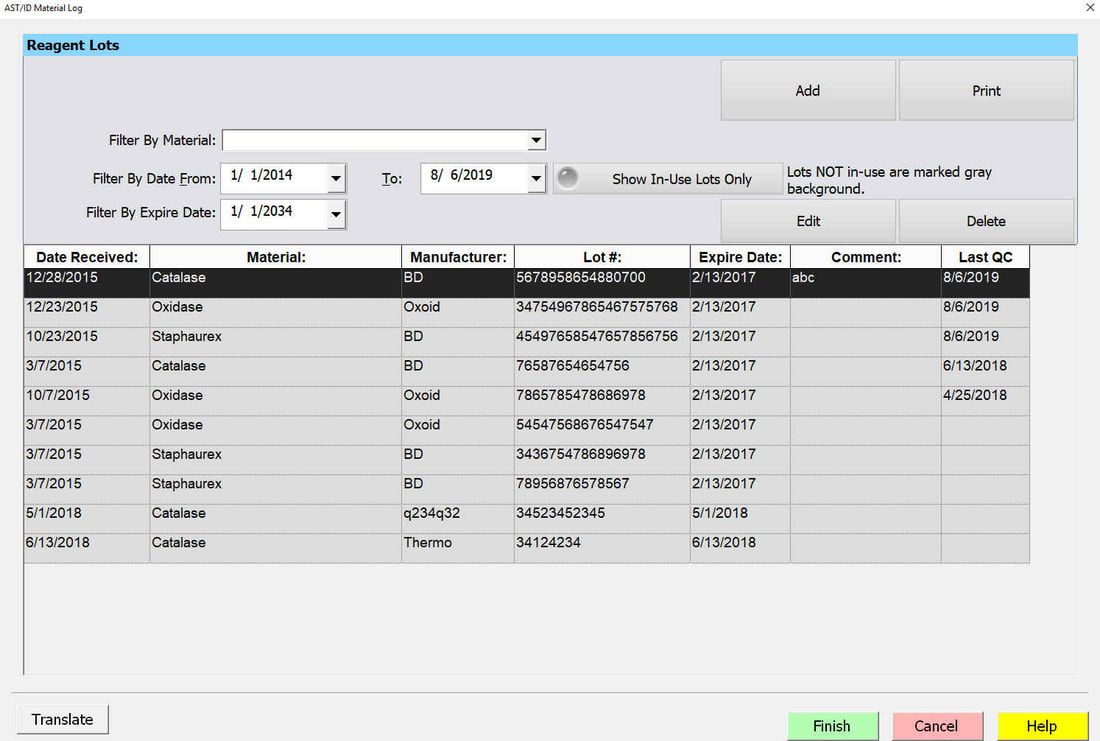
Reagent QC
Reagent QC test profiles (Oxidase, Catalase, PYR, etc) are customized by each lab. Reagent test results and lot information are electronically documented. Reagent QC reports may also be generated.
QC Reports
Custom QC reports and templates may be generated for disk zones, MICs, ID panels, and reagents. All reports include an optional electronic signature review.
|
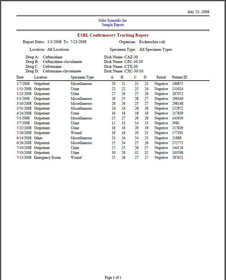
Sample Reports:
|
CAP Checklist
BIOMIC V3 contains all CAP (College of American Pathologists) Bacteriology Checklist Items relevant to BIOMIC V3. This provides advance preparation for laboratory inspections. Examples include:
CAP Checklist Item: MIC.21040 - There is documentation of corrective action when control results exceed defined tolerance limits.
Comments may be recorded for individual disk and Etest QC results outside acceptable CLSI recommended limits. Comments are displayed on QC Summary Reports with Date, Organism, Acceptable Range, Initial, and Measurements.
CAP Checklist Item: MIC.21220 - The laboratory has documentation that each shipment of purchased media is examined for breakage, contamination, appearance, and evidence of freezing or overheating.
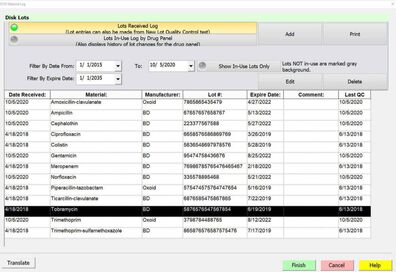
The “Media Lots Received Log” enables recording of: Entry Date, Material, Manufacturer, Lot #, Expiration Date, Comments, Quantity Received, Visual Inspection, Similar Disk, Media, ID Panel and Reagent Logs can also be recorded and printed.
CAP Checklist Item: MIC.21930 - For antimicrobial susceptibility testing systems, there are documented criteria for interpretation of the endpoint or zone size.
Current CLSI zone diameter and QC interpretive guidelines are built into the software. Guideline updates can be downloaded from our website.
CAP Checklist Item: MIC.21040 - There is documentation of corrective action when control results exceed defined tolerance limits.
Comments may be recorded for individual disk and Etest QC results outside acceptable CLSI recommended limits. Comments are displayed on QC Summary Reports with Date, Organism, Acceptable Range, Initial, and Measurements.
CAP Checklist Item: MIC.21220 - The laboratory has documentation that each shipment of purchased media is examined for breakage, contamination, appearance, and evidence of freezing or overheating.
The “Media Lots Received Log” enables recording of: Entry Date, Material, Manufacturer, Lot #, Expiration Date, Comments, Quantity Received, Visual Inspection, Similar Disk, Media, ID Panel and Reagent Logs can also be recorded and printed.
CAP Checklist Item: MIC.21930 - For antimicrobial susceptibility testing systems, there are documented criteria for interpretation of the endpoint or zone size.
Current CLSI zone diameter and QC interpretive guidelines are built into the software. Guideline updates can be downloaded from our website.
Customer Testimonials - QC & Inventory Management
"Excellent QC features with BIOMIC V3"
"All of our micro techs love the new BIOMIC V3. It reads and interprets our Kirby Bauers based on current CLSI guidelines. It reads our Remel RapID panels such as the RapID ANA, RapID NH, RapID NF, etc. BIOMIC V3 is great for QC because it saves us lots of time and all patient information is stored. No more handwriting QC lot numbers, etc… It is well worth a look! "
Suzanne Landry, MT(ASCP)
Microbiology Supervisor
Our Lady of the Lake Regional Medical Center, Baton Rouge, LA, USA
BIOMIC V3 customer since 2008
"All of our micro techs love the new BIOMIC V3. It reads and interprets our Kirby Bauers based on current CLSI guidelines. It reads our Remel RapID panels such as the RapID ANA, RapID NH, RapID NF, etc. BIOMIC V3 is great for QC because it saves us lots of time and all patient information is stored. No more handwriting QC lot numbers, etc… It is well worth a look! "
Suzanne Landry, MT(ASCP)
Microbiology Supervisor
Our Lady of the Lake Regional Medical Center, Baton Rouge, LA, USA
BIOMIC V3 customer since 2008
"BIOMIC V3: What a QC time saver!"
"The BIOMIC V3 is a great backup system to our Vitek. It has standardized the reading and interpretation of our disk-diffusion susceptibilities using current CLSI interpretations. The test results and images are automatically saved in case you need to review a result. We particularly love the BIOMIC QC program. Not only does it read and record our QC but electronically maintains records of all our antibiotic discs and susceptibility test media. No more handwritten QC lot numbers and results. What a time saver!"
Georgia Colasante, MS, MT(ASCP), SM(NRM)
Manager Microbiology
Health Network Laboratory, Allentown, PA, USA
BIOMIC V3 customer since 2006
"The BIOMIC V3 is a great backup system to our Vitek. It has standardized the reading and interpretation of our disk-diffusion susceptibilities using current CLSI interpretations. The test results and images are automatically saved in case you need to review a result. We particularly love the BIOMIC QC program. Not only does it read and record our QC but electronically maintains records of all our antibiotic discs and susceptibility test media. No more handwritten QC lot numbers and results. What a time saver!"
Georgia Colasante, MS, MT(ASCP), SM(NRM)
Manager Microbiology
Health Network Laboratory, Allentown, PA, USA
BIOMIC V3 customer since 2006
"BIOMIC V3: A valued QC tool in the lab"
“We use BIOMIC V3 to efficiently read and record our weekly KB and Etest QC. As a large teaching hospital our volume and therefore our required QC is quite high. BIOMIC V3 saves time and allows for easy and precise record keeping of all the QC. Customer service has been a great help when called and the software updates are timely and help keep the information current and accurate. The software itself is very user friendly. BIOMIC V3 is a valued tool in our laboratory.”
Rachel Harris, MT
University of Florida Health Shands Hospital, Gainesville, FL, USA
BIOMIC V3 customer since 2006
“We use BIOMIC V3 to efficiently read and record our weekly KB and Etest QC. As a large teaching hospital our volume and therefore our required QC is quite high. BIOMIC V3 saves time and allows for easy and precise record keeping of all the QC. Customer service has been a great help when called and the software updates are timely and help keep the information current and accurate. The software itself is very user friendly. BIOMIC V3 is a valued tool in our laboratory.”
Rachel Harris, MT
University of Florida Health Shands Hospital, Gainesville, FL, USA
BIOMIC V3 customer since 2006
"Quick and accurate Etest and QC reading"
"We use the BIOMIC V3 to read and interpret all of our Kirby Bauers and Etests. It does so quickly and accurately. The system is very user-friendly and the touch screen monitor is a great feature. We store all of our disk lot #’s in the computer, so we no longer have to record them on paper. When running our QC panels, we are able to attach all of the lot #’s and expiration dates of the antibiotic disks to the disks tested for each panel. It is a great way to have everything documented and organized for CAP. The CLSI guidelines are always current. Technical Support (Nik) is outstanding. Someone is always there to take your call, and if they cannot help you at that moment, they get back to you in a timely manner. They also listen to feedback and have included some of our suggestions in their software updates. I highly recommend the BIOMIC V3 for accurate and standardized testing, ease-of-use, and excellent technical support."
Andrea LaGram
Microbiology Supervisor
Martin Memorial Medical Center, Stuart, FL, USA
BIOMIC V3 customer since 2010
"We use the BIOMIC V3 to read and interpret all of our Kirby Bauers and Etests. It does so quickly and accurately. The system is very user-friendly and the touch screen monitor is a great feature. We store all of our disk lot #’s in the computer, so we no longer have to record them on paper. When running our QC panels, we are able to attach all of the lot #’s and expiration dates of the antibiotic disks to the disks tested for each panel. It is a great way to have everything documented and organized for CAP. The CLSI guidelines are always current. Technical Support (Nik) is outstanding. Someone is always there to take your call, and if they cannot help you at that moment, they get back to you in a timely manner. They also listen to feedback and have included some of our suggestions in their software updates. I highly recommend the BIOMIC V3 for accurate and standardized testing, ease-of-use, and excellent technical support."
Andrea LaGram
Microbiology Supervisor
Martin Memorial Medical Center, Stuart, FL, USA
BIOMIC V3 customer since 2010
"Standardized KB reading and QC results/image storage with BIOMIC V3"
"We use the BIOMIC V3 to read and interpret all of our Kirby Bauers (KB). We have a big population of Cystic Fibrosis patients in our hospital. We set up most of the isolates from this patient group by KB. BIOMIC V3 makes it much easier and faster to read and interpret these isolates. We sometimes setup up to 40 KB a day and BIOMIC V3 has really made it faster and easier to read. It has been very useful even for the mucoid or difficult to grow organisms. We may have to make a manual adjustment sometimes, but it is still faster and easier to read than manual reading. It also helps us standardize our readings. We also use BIOMIC V3 to read our KB QC. I also like the fact that it stores all the QC, patient readings and images. I can go back and check the image and the reading when needed. I also like and appreciate the great customer service and the yearly CLSI updates."
Tsigereda Tekle MT (ASCP)
Lead Clinical Lab Scientist
Johns Hopkins Hospital, Baltimore, MD, USA
BIOMIC V3 customer since 2010
"We use the BIOMIC V3 to read and interpret all of our Kirby Bauers (KB). We have a big population of Cystic Fibrosis patients in our hospital. We set up most of the isolates from this patient group by KB. BIOMIC V3 makes it much easier and faster to read and interpret these isolates. We sometimes setup up to 40 KB a day and BIOMIC V3 has really made it faster and easier to read. It has been very useful even for the mucoid or difficult to grow organisms. We may have to make a manual adjustment sometimes, but it is still faster and easier to read than manual reading. It also helps us standardize our readings. We also use BIOMIC V3 to read our KB QC. I also like the fact that it stores all the QC, patient readings and images. I can go back and check the image and the reading when needed. I also like and appreciate the great customer service and the yearly CLSI updates."
Tsigereda Tekle MT (ASCP)
Lead Clinical Lab Scientist
Johns Hopkins Hospital, Baltimore, MD, USA
BIOMIC V3 customer since 2010
"Automated Quality Control features for my vet diagnostic lab"
"In addition to its many testing functions, BIOMIC V3 also provides my lab with automated QC testing and electronic tracking of results. Its animal specific options have made BIOMIC V3 a great addition to our vet lab. Their customer support is quick to respond and knowledgeable."
Peggy Dearing
Microbiology Supervisor
Oregon State University-Veterinary Diagnostic Laboratory, Corvallis, OR, USA
BIOMIC V3 customer since 2005
"In addition to its many testing functions, BIOMIC V3 also provides my lab with automated QC testing and electronic tracking of results. Its animal specific options have made BIOMIC V3 a great addition to our vet lab. Their customer support is quick to respond and knowledgeable."
Peggy Dearing
Microbiology Supervisor
Oregon State University-Veterinary Diagnostic Laboratory, Corvallis, OR, USA
BIOMIC V3 customer since 2005